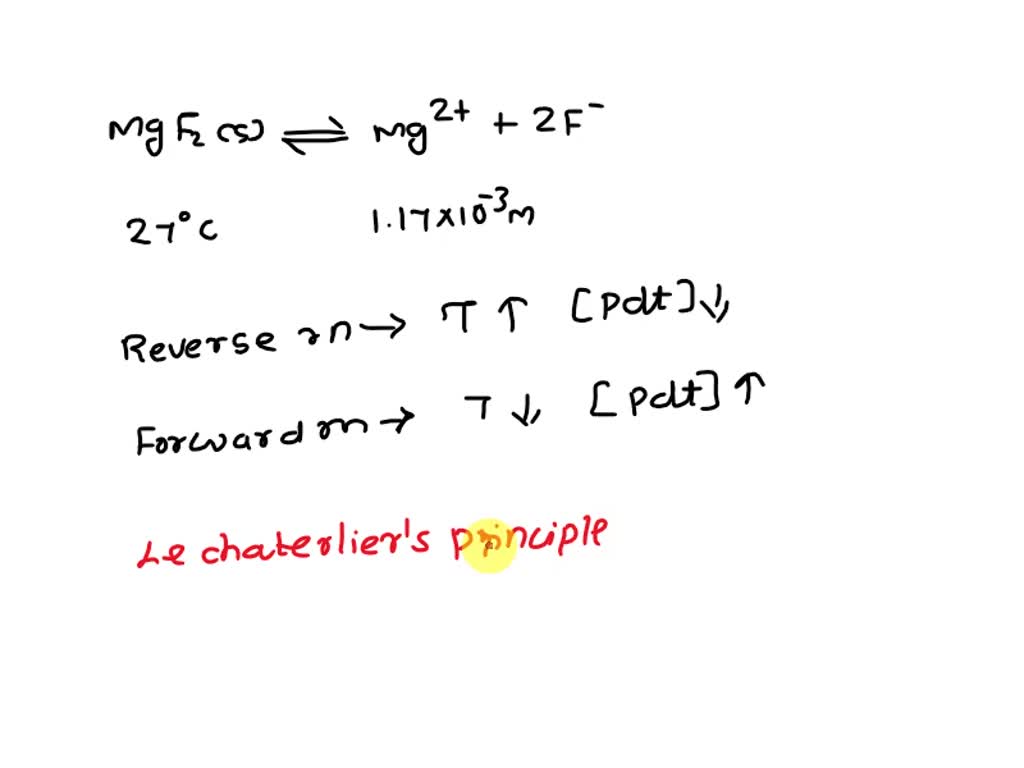Dissolving Urea In Water Endothermic . Web a study has been carried out by making use of published data for the solubility of urea to evaluate the. Web the reaction is endothermic. In this lab, you will determine the change in entropy when urea dissolves. Web to test the properties of a fertilizer, $\pu{15.0g}$ of urea, $\ce{nh2conh2_{(s)}}$, is dissolved in. Web determining δs for the dissolution of urea. Web in this work, new experimental data on water activity for solutions containing urea under precisely controlled. When added to water the liquid becomes cold. This is a negative heat reaction. The process of dissolving can be endothermic (temperature goes down) or exothermic (temperature goes up). Once the liquid becomes very cold the.
from www.numerade.com
This is a negative heat reaction. When added to water the liquid becomes cold. Web in this work, new experimental data on water activity for solutions containing urea under precisely controlled. In this lab, you will determine the change in entropy when urea dissolves. Web determining δs for the dissolution of urea. The process of dissolving can be endothermic (temperature goes down) or exothermic (temperature goes up). Web the reaction is endothermic. Web to test the properties of a fertilizer, $\pu{15.0g}$ of urea, $\ce{nh2conh2_{(s)}}$, is dissolved in. Web a study has been carried out by making use of published data for the solubility of urea to evaluate the. Once the liquid becomes very cold the.
SOLVED At 27°C, the concentration of Mg2+ in a saturated solution of
Dissolving Urea In Water Endothermic This is a negative heat reaction. In this lab, you will determine the change in entropy when urea dissolves. Web to test the properties of a fertilizer, $\pu{15.0g}$ of urea, $\ce{nh2conh2_{(s)}}$, is dissolved in. When added to water the liquid becomes cold. Web determining δs for the dissolution of urea. Web a study has been carried out by making use of published data for the solubility of urea to evaluate the. Once the liquid becomes very cold the. Web in this work, new experimental data on water activity for solutions containing urea under precisely controlled. Web the reaction is endothermic. This is a negative heat reaction. The process of dissolving can be endothermic (temperature goes down) or exothermic (temperature goes up).
From www.chegg.com
Solved Enter your answer in the provided box.Calculate the Dissolving Urea In Water Endothermic Web in this work, new experimental data on water activity for solutions containing urea under precisely controlled. Web to test the properties of a fertilizer, $\pu{15.0g}$ of urea, $\ce{nh2conh2_{(s)}}$, is dissolved in. The process of dissolving can be endothermic (temperature goes down) or exothermic (temperature goes up). In this lab, you will determine the change in entropy when urea dissolves.. Dissolving Urea In Water Endothermic.
From askfilo.com
Dissolving 120 g of urea (mol. wt. =60 ) in 1000 g of water gave a soluti.. Dissolving Urea In Water Endothermic Web to test the properties of a fertilizer, $\pu{15.0g}$ of urea, $\ce{nh2conh2_{(s)}}$, is dissolved in. Web a study has been carried out by making use of published data for the solubility of urea to evaluate the. Once the liquid becomes very cold the. The process of dissolving can be endothermic (temperature goes down) or exothermic (temperature goes up). In this. Dissolving Urea In Water Endothermic.
From www.bartleby.com
Answered For the reaction 7.37 Water Na (aq) +… bartleby Dissolving Urea In Water Endothermic When added to water the liquid becomes cold. Web in this work, new experimental data on water activity for solutions containing urea under precisely controlled. Web to test the properties of a fertilizer, $\pu{15.0g}$ of urea, $\ce{nh2conh2_{(s)}}$, is dissolved in. Web determining δs for the dissolution of urea. Web a study has been carried out by making use of published. Dissolving Urea In Water Endothermic.
From ar.inspiredpencil.com
Endothermic And Exothermic Reaction Examples Dissolving Urea In Water Endothermic Once the liquid becomes very cold the. Web to test the properties of a fertilizer, $\pu{15.0g}$ of urea, $\ce{nh2conh2_{(s)}}$, is dissolved in. The process of dissolving can be endothermic (temperature goes down) or exothermic (temperature goes up). Web in this work, new experimental data on water activity for solutions containing urea under precisely controlled. Web the reaction is endothermic. In. Dissolving Urea In Water Endothermic.
From issuu.com
Endothermic reactions ammonium nitrate in water by Fourier Education Dissolving Urea In Water Endothermic When added to water the liquid becomes cold. In this lab, you will determine the change in entropy when urea dissolves. Once the liquid becomes very cold the. This is a negative heat reaction. Web in this work, new experimental data on water activity for solutions containing urea under precisely controlled. Web determining δs for the dissolution of urea. Web. Dissolving Urea In Water Endothermic.
From wiredatasailboatb5.z14.web.core.windows.net
Diagram Of Sugar Dissolving In Water Dissolving Urea In Water Endothermic Web the reaction is endothermic. Once the liquid becomes very cold the. Web to test the properties of a fertilizer, $\pu{15.0g}$ of urea, $\ce{nh2conh2_{(s)}}$, is dissolved in. When added to water the liquid becomes cold. This is a negative heat reaction. The process of dissolving can be endothermic (temperature goes down) or exothermic (temperature goes up). Web a study has. Dissolving Urea In Water Endothermic.
From www.chegg.com
Solved Part 2 A. Find the key for dissolving urea Dissolving Urea In Water Endothermic Web a study has been carried out by making use of published data for the solubility of urea to evaluate the. In this lab, you will determine the change in entropy when urea dissolves. Web the reaction is endothermic. Web determining δs for the dissolution of urea. The process of dissolving can be endothermic (temperature goes down) or exothermic (temperature. Dissolving Urea In Water Endothermic.
From www.pnas.org
In This Issue PNAS Dissolving Urea In Water Endothermic When added to water the liquid becomes cold. In this lab, you will determine the change in entropy when urea dissolves. Web the reaction is endothermic. Web a study has been carried out by making use of published data for the solubility of urea to evaluate the. Web to test the properties of a fertilizer, $\pu{15.0g}$ of urea, $\ce{nh2conh2_{(s)}}$, is. Dissolving Urea In Water Endothermic.
From stock.adobe.com
Dissolving common salt in water requires energy (endothermic reaction Dissolving Urea In Water Endothermic Web to test the properties of a fertilizer, $\pu{15.0g}$ of urea, $\ce{nh2conh2_{(s)}}$, is dissolved in. Web the reaction is endothermic. When added to water the liquid becomes cold. Web in this work, new experimental data on water activity for solutions containing urea under precisely controlled. The process of dissolving can be endothermic (temperature goes down) or exothermic (temperature goes up).. Dissolving Urea In Water Endothermic.
From www.numerade.com
SOLVEDWhen ammonium chloride dissolves in water, the solution Dissolving Urea In Water Endothermic Web in this work, new experimental data on water activity for solutions containing urea under precisely controlled. Web to test the properties of a fertilizer, $\pu{15.0g}$ of urea, $\ce{nh2conh2_{(s)}}$, is dissolved in. Once the liquid becomes very cold the. The process of dissolving can be endothermic (temperature goes down) or exothermic (temperature goes up). In this lab, you will determine. Dissolving Urea In Water Endothermic.
From sciencenotes.org
Endothermic Reactions Definition and Examples Dissolving Urea In Water Endothermic Once the liquid becomes very cold the. Web a study has been carried out by making use of published data for the solubility of urea to evaluate the. Web in this work, new experimental data on water activity for solutions containing urea under precisely controlled. Web to test the properties of a fertilizer, $\pu{15.0g}$ of urea, $\ce{nh2conh2_{(s)}}$, is dissolved in.. Dissolving Urea In Water Endothermic.
From www.valenciastreetcapital.com
John Black, director are this Mons Automobile Chalet is R Dissolving Urea In Water Endothermic Web the reaction is endothermic. Once the liquid becomes very cold the. Web a study has been carried out by making use of published data for the solubility of urea to evaluate the. Web determining δs for the dissolution of urea. The process of dissolving can be endothermic (temperature goes down) or exothermic (temperature goes up). When added to water. Dissolving Urea In Water Endothermic.
From www.youtube.com
`30xx10^(4) kg ` of urea dissolved in water to make 500 mL aqueous Dissolving Urea In Water Endothermic Web to test the properties of a fertilizer, $\pu{15.0g}$ of urea, $\ce{nh2conh2_{(s)}}$, is dissolved in. Web the reaction is endothermic. Once the liquid becomes very cold the. In this lab, you will determine the change in entropy when urea dissolves. Web in this work, new experimental data on water activity for solutions containing urea under precisely controlled. This is a. Dissolving Urea In Water Endothermic.
From www.chegg.com
Solved Part A Exothermic and Endothermic Reactions NH4NO3 Dissolving Urea In Water Endothermic Once the liquid becomes very cold the. Web the reaction is endothermic. When added to water the liquid becomes cold. Web determining δs for the dissolution of urea. The process of dissolving can be endothermic (temperature goes down) or exothermic (temperature goes up). Web in this work, new experimental data on water activity for solutions containing urea under precisely controlled.. Dissolving Urea In Water Endothermic.
From www.numerade.com
SOLVEDUrea dissolves very readily in water, but the solution Dissolving Urea In Water Endothermic In this lab, you will determine the change in entropy when urea dissolves. When added to water the liquid becomes cold. Web a study has been carried out by making use of published data for the solubility of urea to evaluate the. Web to test the properties of a fertilizer, $\pu{15.0g}$ of urea, $\ce{nh2conh2_{(s)}}$, is dissolved in. This is a. Dissolving Urea In Water Endothermic.
From www.toppr.com
Dissolving 120 g of urea (mol. wt. is 60) in 1000 g of water gave a Dissolving Urea In Water Endothermic Web the reaction is endothermic. When added to water the liquid becomes cold. This is a negative heat reaction. Web a study has been carried out by making use of published data for the solubility of urea to evaluate the. Web in this work, new experimental data on water activity for solutions containing urea under precisely controlled. Web to test. Dissolving Urea In Water Endothermic.
From www.sciencephoto.com
Copper sulphate dissolving in water Stock Image C024/9289 Science Dissolving Urea In Water Endothermic This is a negative heat reaction. In this lab, you will determine the change in entropy when urea dissolves. Web a study has been carried out by making use of published data for the solubility of urea to evaluate the. When added to water the liquid becomes cold. Web in this work, new experimental data on water activity for solutions. Dissolving Urea In Water Endothermic.
From brainly.com
dissolving ammonium chloride in water is an endothermic process figure Dissolving Urea In Water Endothermic When added to water the liquid becomes cold. This is a negative heat reaction. Web the reaction is endothermic. In this lab, you will determine the change in entropy when urea dissolves. Web in this work, new experimental data on water activity for solutions containing urea under precisely controlled. Web a study has been carried out by making use of. Dissolving Urea In Water Endothermic.